Neurostar TMS | Magnetic Stimulation Treatment for Depression
NeuroStar TMS Therapy is the first non-systemic and non-invasive outpatient depression treatment cleared by the US Food and Drug Administration (FDA) for patients who have not benefited from prior antidepressant treatment. NeuroStar TMS Therapy uses highly focused, pulsed magnetic fields to stimulate function in targeted brain regions.
NeuroStar TMS Therapy is:
● Non-invasive, meaning that it does not involve surgery. It does not require any anesthesia or sedation, as the patient remains awake and alert during the treatment.
● Non-systemic, meaning that it is not taken by mouth and does not circulate in the bloodstream throughout the body.
The exact cause of depression is unknown, but leading research in Neuroscience points to an imbalance in the brain’s neurotransmitters as the manifestation of depression. Neurotransmitters are chemical messengers that send signals between brain cells. A person’s genetic make-up and life history may also determine a person’s tendency to become depressed.
What is NeuroStar® TMS Therapy?
TMS stands for transcranial magnetic stimulation. It is used to treat depression by stimulating the brain non-invasively using electromagnetic fields, similar to those produced by an MRI machine. During TMS Therapy, a magnetic field is administered in very short pulses to the part of the brain that research has demonstrated to be associated with depression. The typical initial course of treatment is about 19-37 minutes daily over 4-6 weeks.
How does NeuroStar® TMS Therapy work?
The NeuroStar TMS Therapy system uses short pulses of magnetic fields to stimulate the area of the brain that is thought to function abnormally in patients with depression. The magnetic field produces an electric current in the brain that stimulates the brain cells (neurons). This results in changes that are thought to be beneficial in the treatment of depression.
NeuroStar® TMS Therapy | What to expect?
A NeuroStar® TMS Therapy treatment session is a short outpatient procedure that lasts about 19-37 minutes, depending on what the doctor determines is the correct protocol. During treatment, you can relax in the treatment chair. You can also speak with our TMS Specialists whenever necessary. After the procedure, you can immediately return to your normal routine, including driving.
Your First Treatment Session
Because your TMS-certified physician needs to determine how to most effectively administer treatment, your first session could last up to an hour and a half. You will be provided and asked to wear protective earplugs, as the system emits a tapping sound during operation.
Your TMS physician will first perform a test to identify your motor threshold. The motor threshold is the amount of magnetic field strength that results in a movement of your right thumb. This test is important because it identifies the magnetic field strength that will be used in your treatment. This field strength is customized for each patient to deliver the correct treatment dose.
The TMS physician will determine the place on the head where the TMS treatment will be applied and the magnetic coil will be moved to that location. TMS Therapy will then be administered over the next 19-37 minutes, approximately.
In 10-second intervals, the device will deliver rapid “pulses” of the magnetic fields. These will feel like tapping on your scalp. Some patients may find this tapping uncomfortable. Your physician may be able to make adjustments to reduce this discomfort.
While there may be some minor discomfort at the treatment site (where the device touches your head), it generally subsides within the first week or treatment. There is no sedation, or impact on your alertness. You can read, watch TV, or talk with your treatment coordinator during your session, and you can drive home immediately after treatment.
Does it hurt?
While there may be some minor discomfort at the treatment site (where the device touches your head), it generally subsides within the first week or treatment. There is no sedation, or impact on your alertness. You can read, watch TV, or talk with your treatment coordinator during your session, and you can drive home immediately after treatment.
NeuroStar® TMS Therapy | What are possible side effects?
Immediately following each treatment session, you may return to your normal daily routine, including driving. During or after treatment you may experience a headache or discomfort at the site of stimulation. These are common side effects that often improve as further treatment sessions are administered. If necessary, you can treat this discomfort with an over-the-counter analgesic.
If these side effects persist, your TMS physician can temporarily reduce the strength of the magnetic field pulses being administered in order to make treatment more comfortable.
NeuroStar® TMS Therapy | When will I see results?
In clinical trials, most patients who benefited from NeuroStar TMS Therapy experienced results by the fourth week of treatment. Some patients may experience results in less time, while others may take longer. You should discuss your depression symptoms with your physician throughout the treatment course. If symptoms persist, you may want to consider other antidepressant options.
NeuroStar® TMS Therapy | Scientific Reviews & Efficacy Studies
Clinical trials have demonstrated the effectiveness of NeuroStar® TMS Therapy in treating patients who have not benefited from prior antidepressant medication. NeuroStar TMS Therapy was studied in adult patients suffering from Major Depressive Disorder, all of whom had not received satisfactory improvement with previous treatments.
In an independent, randomized, controlled trial funded by the National Institute of Mental Health, 307 patients were treated with the NeuroStar TMS System for 4 to 6 weeks, similar to real clinical context.1
Patients were divided into two groups:
- Low Treatment Resistance: Patients who have failed to improve their depression symptoms after a single antidepressant treatment of adequate dose and duration.
- High Treatment Resistance: Patients who have failed to improve their depression symptoms after multiple (2-14) antidepressant treatments of adequate dose and duration.
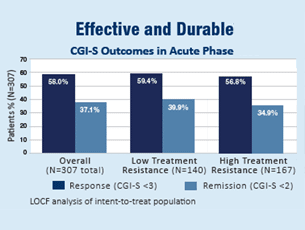
At the end of their treatments, patients who had received NeuroStar TMS Therapy were four times more likely to achieve remission compared to patients receiving a sham treatment. 1 in 2 patients experienced significant improvement in their depression symptoms, and 1 in 3 experienced complete remission. Patients treated with the NeuroStar TMS System also experienced significant improvement in anxiety and physical symptoms (such as appetite changes, aches and pains, and lack of energy) associated with depression.1
NeuroStar® TMS Therapy | Is my treatment covered by insurance?
Since the FDA clearance of TMS in 2008, insurance coverage for eligible patients has increased significantly. Currently, there are over 60 coverage policies for TMS, including most Medicare contractors. Although TMS is not the first line of treatment, it is an alternative option for those who are not responding to or cannot tolerate medications. Patients are encouraged to speak directly with their Doctor regarding any specific insurance questions. Patients can also contact a Reimbursement Specialist and receive assistance with understanding insurance coverage and verifying insurance benefits. For a list of the insurance companies and applicable states that cover TMS Therapy, please click here.
NeuroStar® TMS Therapy | Available Near Me in Arlington Heights, IL
If you are interested in learning more about the ways NeuroStar® TMS Therapy can alleviate symptoms of depression, contact the front desk at Riverside Medical, S.C. by calling (847) 577-9300 or by completing the form below to schedule your no-cost consultation.
As a leading provider of comprehensive health and aesthetic services in the Arlington Heights area, our medical clinic has earned its reputation for commitment to providing innovative and effective treatments for our clients, helping them progress to overall wellness.

*By submitting this form, I consent to be contacted by Emerson Medical via phone, text, email, or mail. Message and data rates may apply. Consent is not a condition of purchase. I agree to the Privacy Policy. Reply STOP to opt out.

*By submitting this form, I consent to be contacted by Emerson Medical via phone, text, email, or mail. Message and data rates may apply. Consent is not a condition of purchase. I agree to the Terms of Service and acknowledge the Privacy Policy. Reply STOP to opt out.